Diastereoselective construction of carbazole-based spirooxindoles via the Levy three-component reaction†
Abstract
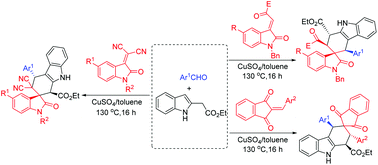
The CuSO4 catalyzed three-component reaction of indole-2-acetate, aromatic aldehydes and 3-methyleneoxindoles in toluene at 130 °C afforded polysubstituted spiro[carbazole-3,3′-indolines] in good yields and with high diastereoselectivity. When isatylidene malononitriles were used as dienophiles, regio-isomeric spiro[carbazole-2,3′-indolines] were selectively obtained. A similar three-component reaction with 2-arylidene-1,3-indanediones resulted in polysubstituted spiro[carbazole-3,2′-indenes] in satisfactory yields and with high diastereoselectivity. The stereochemistry of the diastereoisomers of the spiro compounds was clearly elucidated by analysis of NMR spectra and determination of fourteen single crystal structures. The reaction mechanism included formation of reactive 2,3-dimethyleneindoline and a sequential Diels–Alder reaction.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...