Theoretical insights into the E1cB/E2 mechanistic dichotomy of elimination reactions†
Abstract
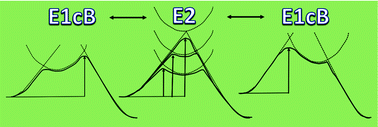
E1cB and E2 eliminations have been described as competing mechanisms that can even share a common pathway when the E1cB/E2 borderline mechanism operates. A suitable case study evincing such a mechanistic dichotomy corresponds to the elimination reaction of β-phenylmercaptoethyl phenolate, since its mechanism has been thought to be an E2 elimination. Nonetheless, according to the computational assessment of the substituents on the leaving group, we demonstrate that the reaction proceeds via a borderline E1cB mechanism. Stabilization of the carbanion was provided not only by substituent effects tuning the nucleofugality of the leaving group, but also by a base, since distortion/interaction–activation strain and Natural Bond Order (NBO) analyses suggest a stabilizing interaction between the base and Cβ of the E1cB intermediate. In order to gain insights into these results in a more general context, we have rationalized them with a qualitative picture of the E1cB/E2 mechanistic dichotomy using simple relationships between diabatic parabolas modeling the potential wells of reactants, intermediates, and products. In this Diabatic Model of Intermediate Stabilization (DMIS), the borderline E1cB mechanism for the elimination reaction of β-phenylmercaptoethyl phenolate was discussed in terms of bonding and dynamic stepwise processes. The conceptual model presented herein should be useful for the analysis of any reaction comprising competing one- and two-step mechanisms.
- This article is part of the themed collections: Celebrating Latin American Talent in Chemistry and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...