Studies toward the synthesis of macrotermycin C: stereoselective construction of the acyclic skeleton of the aglycon†
Abstract
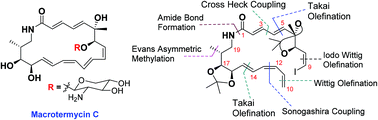
The stereoselective construction of the acyclic skeleton of the aglycon of polyene macrolactam macrotermycin C has been achieved for the first time using a convergent strategy. The important features of this synthesis study include Evans methylation, Takai olefination, Sonogashira coupling followed by selective alkyne reduction, intermolecular Heck coupling, and Wittig olefination. Intramolecular Heck coupling has been tested for macrocyclization.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...