Abstract
A Rh-catalyzed domino synthesis of 4-hydroxy-3-methylcoumarins via branch-selective hydroacylation of acrylates and acrylamides using salicylaldehydes is described. This protocol under phosphine-free Rh-catalyzed conditions provided 4-hydroxy-3-methylcoumarins in high yields with excellent functional group tolerance and high selectivity.
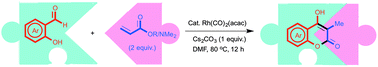
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...