The ruthenium-catalyzed meta-selective C–H nitration of various azole ring-substituted arenes†
Abstract
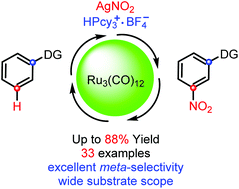
The efficient ruthenium-catalyzed meta-selective CAr–H nitration of azole ring substituted arenes has been developed. In this work, Ru3(CO)12 was used as the catalyst, AgNO2 as the nitro source, HPcy3+·BF4− as the ligand, pivalic acid as the additive, and DCE as the solvent, and a wide spectrum of arenes bearing thiazole, pyrazolyl or removable oxazoline directing groups were tolerated in this meta-selective CAr–H nitration, affording the nitrated products in moderate to good yields. Moreover, this study reveals a gentler and environmentally friendly way to access meta-nitration arenes compared to the traditional process.

 Please wait while we load your content...
Please wait while we load your content...