Abstract
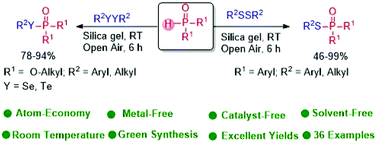
Silica gel promoted, catalyst-free and solvent-free S–P, Se–P and Te–P bond formations are described. A variety of disulfides coupled with diarylphosphine oxides provide the corresponding phosphinothioates in excellent yields. For the first time, diselenides and ditellurides reacted with dialkyl phosphites under catalyst-free conditions to provide the corresponding phosphoroselenoates and phosphorotelluroates, respectively, in good to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...