Transition-metal free C(sp2)–C(sp2) bond formation: arylation of 4-aminocoumarins using arynes as an aryl source†
Abstract
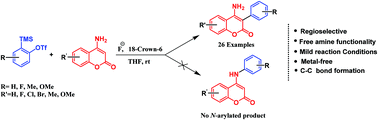
A mild, efficient and transition-metal free synthetic strategy has been developed for the α-arylation of 4-aminocoumarins. This synthetic strategy proceeds via C(sp2)–C(sp2) bond formation between 4-aminocoumarins and aryne precursors in a single step by simple treatment with a fluoride source in the absence of a metal-catalyst. Moreover, this methodology affords good yields of 4-amino-3-arylcoumarin derivatives bearing halide functionality.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...