Divergent pathway and reactivity control of intramolecular arene C–H vinylation by vinyl cations†
Abstract
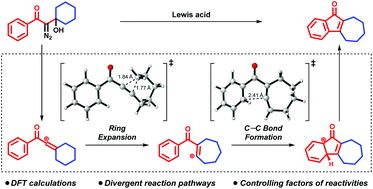
Vinyl cations exhibit remarkable reactivity towards arene C–H functionalizations. This computational study revealed the key mechanistic details of intramolecular C–H vinylation through a vinyl cation intermediate. Based on the reaction mechanism, the effects of substitution, ring strain and tether length on the reactivity of the vinyl cation were elucidated.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...