Syntheses of doubly linked proanthocyanidins using free flavan units as nucleophiles: insight into the origin of the high regioselectivity of annulation†
Abstract
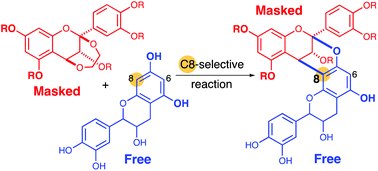
A synthesis method of doubly linked flavan dimers is reported via the acid-promoted annulation reaction using nascent catechins, (+)-catechin or (−)-epicatechin, as a dianionic partner and an ethylenedioxy-bridged flavan as a dicationic partner. Procyanidins A1 and A2 were synthesized. On the high regioselectivity of the annulation reactions, model experiments and computational studies were carried out.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...