From ferrocene to fluorine-containing penta-substituted derivatives and all points in-between; or, how to increase the available chemical space†
Abstract
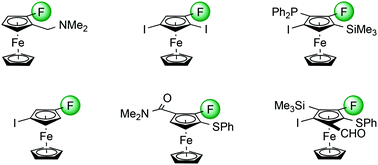
In spite of the growing interest in fluorine-containing compounds, and the improvements in materials, optical and biological properties that can arise from substitution of a phenyl ring by ferrocene within a molecular scaffold, synthetic strategies that allow the efficient preparation of fluoroferrocene derivatives are scarce. Following conversion of ferrocene to fluoroferrocene, we have developed routes to fluorine-containing di-, tri-, tetra- and penta-substituted ferrocene derivatives to extend the available chemical space. Our approach is based on the identification of suitable reagents and conditions to achieve fluorine-directed deprotometalation, and exploitation of the halogen ‘dance’ rearrangement in the ferrocene series.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...