Synthesis of 3-(tri/difluoromethyl)-1H-1,2,4-triazol-5(4H)-ones via the cyclization of hydrazinecarboxamides with tri/difluoroacetic anhydride†
Abstract
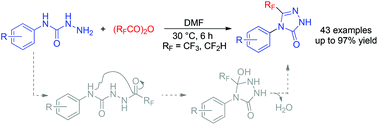
An efficient method for the synthesis of structurally diverse 4-aryl-3-(tri/difluoromethyl)-1H-1,2,4-triazol-5(4H)-ones through the cyclization of hydrazinecarboxamides with tri/difluoroacetic anhydride is presented. The method is simple and environmentally benign, providing tri/difluoromethylated 1,2,4-triazol-5(4H)-ones in moderate-to-good yields. A mechanism is proposed to proceed via a tandem reaction of tri/difluoroacetylation, nucleophilic addition and water elimination. Some of these compounds exhibit promising insecticidal activities.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...