Substrate-product analogue inhibitors of isoleucine 2-epimerase from Lactobacillus buchneri by rational design†
Abstract
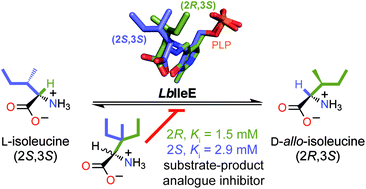
A rational approach that may be applied to a broad class of enzyme-catalyzed reactions to design enzyme inhibitors affords a powerful strategy, facilitating the development of drugs and/or molecular probes of enzyme mechanisms. A strategy for the development of substrate-product analogues (SPAs) as inhibitors of racemases and epimerases is elaborated using isoleucine 2-epimerase from Lactobacillus buchneri (LbIleE) as a model enzyme. LbIleE catalyzes the PLP-dependent, reversible, racemization or epimerization of nonpolar amino acids at the C-2 position. The enzyme plays an important role in the biosynthesis of branched-chain D-amino acids and is a potential target for the development of antimicrobial agents. 3-Ethyl-3-methyl-L-norvaline (Ki = 2.9 ± 0.2 mM) and 3-ethyl-3-methyl-D-norvaline (Ki = 1.5 ± 0.2 mM) were designed as SPAs based on the movement of the sec-butyl side chain of the substrate L-Ile during catalysis, and were competitive inhibitors with binding affinities exceeding that of L-Ile by 1.3- and 2.5-fold, respectively. Surprisingly, these compounds were not substrates, but the corresponding compounds lacking the 3-methyl group were substrates. Unlike serine, glutamate, and proline racemases, which exhibit stringent steric requirements at their active sites, the active site of LbIleE was amenable to binding bulky SPAs. Moreover, LbIleE bound the SPA 2,2-di-n-butylglycine (Ki = 11.0 ± 0.2 mM) as a competitive inhibitor, indicating that the hydrophobic binding pocket at the active site was sufficiently plastic to tolerate gem-dialkyl substitution at the α-carbon of an amino acid. Overall, these results reveal that amino acid racemases/epimerases are amenable to inhibition by SPAs provided that they possess a capacious active site.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...