Correlating ionic liquid solvent effects with solvent parameters for a reaction that proceeds through a xanthylium intermediate†
Abstract
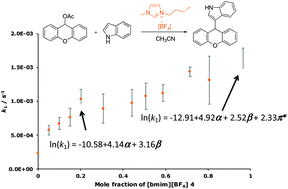
A unimolecular nucleophilic substitution reaction that proceeds through a xanthylium carbocation was studied in seven ionic liquid solvents. It was found that the general trend in the rate constant with changing proportion of ionic liquid in the reaction mixture was different to that seen for other unimolecular processes, with the rate constant increasing as more ionic liquid was added to the reaction mixture. A significant correlation was found between the natural logarithm of the rate constant and a combination of the Kamlet–Taft solvent parameters. This relationship indicated that the principal interaction involved hydrogen bonding between the ionic liquid and some species along the reaction coordinate. Further, this correlation enables prediction of the effects that other ionic liquids will have on this, and other, reactions that proceed through a similar intermediate.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...