Impact of substituent effects on the design of β-sheet mimetics and β-double helices from (E)-vinylogous γ-amino acid oligomers†
Abstract
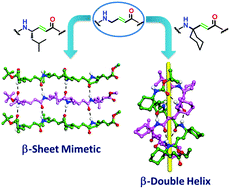
Here we report the design, single crystal conformations, impact of the substituents and structural differences of two structurally important motifs, β-sheets and β-double helices. Though β-sheets are common structural motifs in protein structures, β-double helices are not common in proteins and peptides. We found that both β-sheet mimetics and β-double helices can be constructed from the homooligomers of α,β-unsaturated γ-amino acids. Results suggested that introducing gem-dialkyl substitutions at the γ-carbon of the homooligomer of α,β-unsaturated γ-amino acids resulted in the β-double helix conformation, while the same oligomer with monosubstitution at the γ-carbon displayed a β-sheet structure.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...