cis-Selective synthesis of 1,3-disubstituted tetrahydro-β-carbolines from N-sulfonyl N,S-acetals†
Abstract
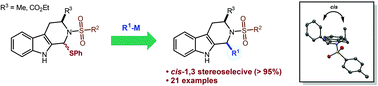
Nucleophilic addition of Grignard reagents to tetrahydro-β-carboline (THC) N-sulfonyl N,S-acetal generates exclusively cis-1,3-disubstituted THCs with a unique 1,3-diaxial conformation. The stereochemical relationship of the 1,3-substituents was confirmed by 2-dimensional NMR spectroscopy and X-ray crystallography. The mechanism of the reaction is proposed based on crystal structures and molecular orbital calculations.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...