Selective inhibition of APOBEC3 enzymes by single-stranded DNAs containing 2′-deoxyzebularine†
Abstract
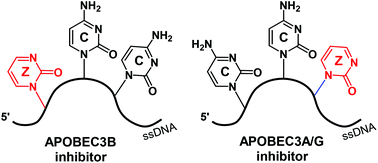
To restrict pathogens, in a normal human cell, APOBEC3 enzymes mutate cytosine to uracil in foreign single-stranded DNAs. However, in cancer cells, APOBEC3B (one of seven APOBEC3 enzymes) has been identified as the primary source of genetic mutations. As such, APOBEC3B promotes evolution and progression of cancers and leads to development of drug resistance in multiple cancers. As APOBEC3B is a non-essential protein, its inhibition can be used to suppress emergence of drug resistance in existing anti-cancer therapies. Because of the vital role of APOBEC3 enzymes in innate immunity, selective inhibitors targeting only APOBEC3B are required. Here, we use the discriminative properties of wild-type APOBEC3A, APOBEC3B and APOBEC3G to deaminate different cytosines in the CCC-recognition motif in order to best place the cytidine analogue 2′-deoxyzebularine (dZ) in the CCC-motif. Using several APOBEC3 variants that mimic deamination patterns of wild-type enzymes, we demonstrate that selective inhibition of APOBEC3B in preference to other APOBEC3 constructs is feasible for the dZCC motif. This work is an important step towards development of in vivo tools to inhibit APOBEC3 enzymes in living cells by using short, chemically modified oligonucleotides.


 Please wait while we load your content...
Please wait while we load your content...