Understanding the effects of solvate ionic liquids as solvents on substitution processes†
Abstract
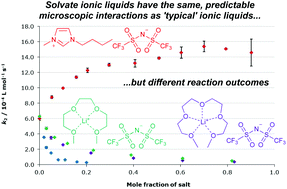
The effects of solvate ionic liquids as solvents have been considered for two substitution processes where the solvent effects of typical ionic liquids have been extensively investigated previously; the bimolecular nucleophilic substitution (SN2) reaction between pyridine and benzyl bromide and the nucleophilic aromatic substitution (SNAr) reaction between ethanol and 1-fluoro-2,4-dinitrobenzene. It was found that use of solvate ionic liquids gave rise to similar trends in the activation parameters for both substitution processes as typical ionic liquids, implying the microscopic interactions responsible for the effects were the same. However, different effects on the rate constants compared to typical ionic liquids were observed due to the changes in the balance of enthalpic and entropic contributions to the observed rate constants. From these data it is clear that the reaction outcome for both of these substitution reactions fall within the ‘predictive framework’ established in previous studies with a cautionary tale or two of their own to add to the general knowledge of ionic liquid solvent effects for these processes, particularly with respect to potential reactivity of the solvate ionic liquids themselves.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...