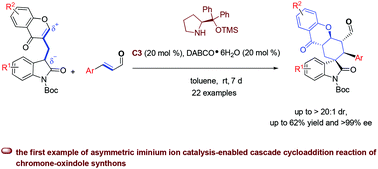
An asymmetric iminium ion catalysis-enabled cascade cycloaddition reaction of chromone-oxindole synthons with enals: construction of a spirooxindole–hexahydroxanthone framework†
Abstract
We report the first asymmetric iminium ion catalysis-enabled cascade cycloaddition reaction of bifunctional chromone-oxindole synthons and α,β-unsaturated aldehydes. This allowed one quaternary and four tertiary contiguous stereogenic centers to be constructed in a single operation. A range of spirooxindole–hexahydroxanthone molecules are obtained with up to 62% yield, >20 : 1 dr and >99% ee. This reaction has not only provided a new approach for constructing hexahydroxanthone-fused scaffolds by utilizing asymmetric iminium ion catalysis, but also advanced the chemistry of diversity-oriented synthesis based on bifunctional chromone synthons.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...