Expedient synthesis of highly substituted 3,4-dihydro-1,2-oxathiine 2,2-dioxides and 1,2-oxathiine 2,2-dioxides: revisiting sulfene additions to enaminoketones†
Abstract
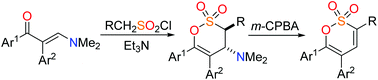
Diversely substituted 1,2-oxathiine 2,2-dioxides, including 3,5,6-triaryl-, 3,6-diaryl-, 3,5-diaryl-, 5,6-diaryl- and selected fused heterocyclic analogues, have been efficiently obtained by the application of a mild Cope elimination of a 4-amino moiety from the requisite 4-amino-3,4-dihydro-1,2-oxathiine 2,2-dioxides, which themselves were readily obtained by the addition of sulfenes to enaminoketones.


 Please wait while we load your content...
Please wait while we load your content...