Chiral bisphosphine ligands based on quinoline oligoamide foldamers: application in asymmetric hydrogenation†
Abstract
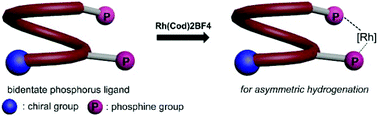
A series of chiral bisphosphine ligands were designed and synthesized based on single-handed quinoline oligoamide foldamers. The bisphosphine ligands can coordinate with Rh(cod)2BF4 in a 1 : 1 stoichiometry and the resulted chiral Rh(I) catalysts were applied in the asymmetric hydrogenation of α-dehydroamino acid esters, in which excellent conversions and promising levels of enantioselectivity were achieved.
- This article is part of the themed collections: Catalysis & biocatalysis in OBC and Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...