Application of copper(i) salt and fluoride promoted Stille coupling reactions in the synthesis of bioactive molecules
Abstract
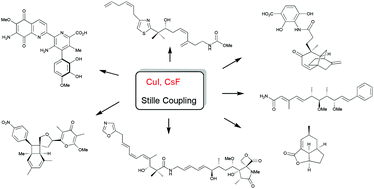
The Stille coupling between organostannanes and organohalides is an effective catalytic method for organic synthesis. Despite the ample amount of published results in this area, finding the optimal conditions for this transformation is often not straightforward. It was observed that this reaction could be accelerated with improved efficiency by the addition of a Cu(I) salt and fluoride. This review summarises the application of this simple protocol in the synthesis of natural products, their analogues and other biologically active molecules, from 2004 to 2018.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...