The one pot asymmetric synthesis of 3,3′-pyrrolidonyl spiroxindoles via a regio- and stereoselective domino reaction†
Abstract
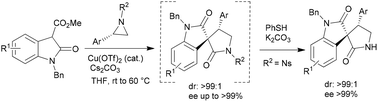
A highly efficient regio- and stereoselective domino aziridine ring opening and lactamization reaction between aziridines and 3-carboxy oxindole esters has been developed for the one pot asymmetric synthesis of 4-aryl-3,3′-spiropyrrolidonyl oxindoles with excellent selectivity (dr >99 : 1 and ee up to >99%). It was further extended to a sequential one pot protocol for the asymmetric synthesis of NH-free 3,3′-pyrrolidonyl spiroxindole, maintaining the same selectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...