Stereodivergent synthesis of alkenes by controllable syn-/anti-fragmentation of β-hydroxysulfonyl intermediates†
Abstract
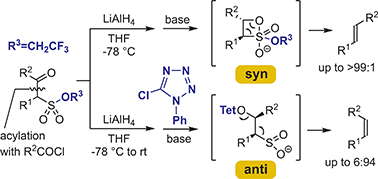
The reduction of the carbonyl group in acylated trifluoroethyl alkanesulfonates follows the Felkin–Ahn selectivity, and the so-formed diastereomeric β-hydroxysulfonyl intermediates undergo syn- and anti-fragmentation, depending on the reaction conditions. In effect, isomeric E- and Z-alkenes are formed in a stereodivergent manner, which mimics the mechanistic manifold of the Peterson olefination.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...