The Mukaiyama type aldol reaction for the synthesis of trans-2,6-disubstituted tetrahydropyrans: synthesis of diospongin A and B†
Abstract
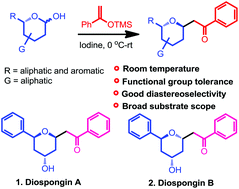
An efficient synthesis protocol for the preparation of trans-2,6-disubstituted tetrahydropyrans by the reaction of 1-phenyl-1-triemthylsiloxyethylene with six membered cyclic hemiacetals in the presence of iodine is developed. This reaction proceeds smoothly under mild conditions employing a catalytic amount of molecular iodine. The feature of this novel conversion includes milder reaction conditions, broader substrate scope, functional group tolerance and good diastereoselectivity. The efficiency and practicality of the current method were successfully displayed in the total synthesis of diospongin A and B in good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...