Norbornadiene–dihydroazulene conjugates†
Abstract
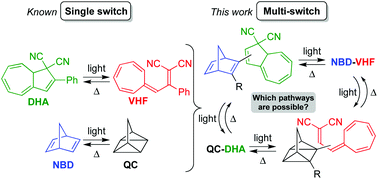
The introduction of various photochromic units into the same molecule is an attractive approach for the development of novel molecular solar thermal (MOST) energy storage systems. Here, we present the synthesis and characterisation of a series of covalently linked norbornadiene/dihydroazulene (NBD/DHA) conjugates, using the Sonogashira coupling as the key synthetic step. Generation of the fully photoisomerized quadricyclane/vinylheptafulvene (QC/VHF) isomer was found to depend strongly on how the two units are connected – by linear conjugation (a para-phenylene bridge) or cross-conjugation (a meta-phenylene bridge) or by linking to the five- or seven-membered ring of DHA – as well as on the electronic character of another substituent group on the NBD unit. When the QC–VHF system could be reached, the QC-to-NBD back-reaction occurred faster than the VHF-to-DHA back-reaction, while the latter could be promoted simply by the addition of Cu(I) ions. The absence or presence of Cu(I) can thus be used to control whether heat releases should occur on different or identical time scales. The experimental findings were rationalized in a computational study by comparing natural transition orbitals (NTOs). Moreover, the calculations revealed an energy storage capacity of 106–110 kJ mol−1 of the QC–VHF isomers, which is higher than the sum of the capacities of the individual, separate units. The major contribution to the energy storage relates to the energetic QC form, while the major contribution to the absorption of visible light originates from the DHA photochrome; some of the NBD–DHA conjugates had absorption onsets at 450 nm or beyond.


 Please wait while we load your content...
Please wait while we load your content...