Direct methyl C(sp3)–H azolation of thioanisoles via oxidative radical coupling†
Abstract
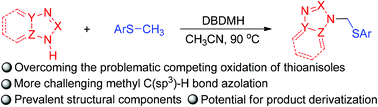
A method for metal-free, 1,3-dibromo-5,5-dimethylhydantoin mediated methyl C(sp3)–H bond azolation of thioanisoles has been developed, affording a facile route for the construction of nitrogen-functionalized thioanisoles, possibly via a nitrogen-centered radical process. This reaction represents an important addition to the limited number of existing methods for the methyl C(sp3)–H bond functionalization of thioanisoles, and may find practical application in the synthesis of nitrogen-alkylated azoles.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...