Thermodynamically driven self-assembly of pyridinearene to hexameric capsules†
Abstract
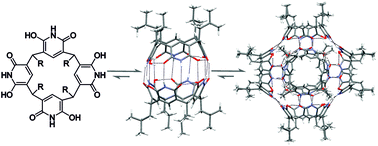
Pyridinearene macrocycles have previously shown unique host–guest properties in their capsular dimers including endo complexation of neutral molecules and exo complexation of anions. Here, we demonstrate for the first time the formation of hydrogen bonded hexamer of tetraisobutyl-octahydroxypyridinearene in all three states of matter – gas phase, solution and solid-state. Cationic tris(bipyridine)ruthenium(II) template was found to stabilize the hexamer in gas phase, whereas solvent molecules do this in condensed phases. In solution, the capsular hexamer was found to be the thermodynamically favoured self-assembly product and transition from dimer to hexamer occurred in course of time. The crystal structure of hexamer revealed 24 N–H⋯O direct intermolecular hydrogen bonds between the six pyridinearene macrocycles without any bridging solvent molecules. Hydrogen bond patterns correlate well with DFT computed structures. Thus, all structural chemistry methods (IM-MS, DOSY NMR, DFT, X-ray crystallography) support the same structure of the hexameric capsule that has a diameter of ca. 3 nm and volume of 1160 Å3.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...