A styryl based fluorogenic probe with high affinity for a cyclodextrin derivative†
Abstract
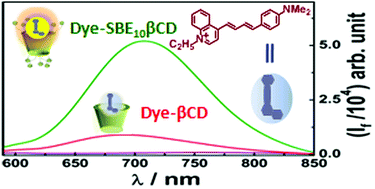
Supramolecular host–guest pairs, with a very high association constant, represent excellent molecular recognition motifs, and serve as useful building blocks for numerous exciting supramolecular functional materials. Cyclodextrins, an important class of glucopyranose-based hosts, generally, suffer from low affinity for most guest molecules (102–104 M−1). Herein, we report a styryl-based fluorogenic probe which registers a very high association constant of 7.84 × 105 M−1 with sulfobutylether substituted β-cyclodextrin in contrast to a low association constant of 1.89 × 102 M−1 with unmodified β-cyclodextrin (β-CD). This exceptionally high binding affinity of the fluorogenic probe with the sulfobutylether substituted β-CD has been attributed to the strong electrostatic interactions between the cationic guest and the polyanionic host along with improved hydrophobic interactions due to the extended butylether groups present on the rims of the cyclodextrin cavity. The significant modulations in the photophysical properties of guest molecule, upon interaction with host molecule, have been investigated, in detail, using ground-state absorption, steady-state fluorescence and time-resolved emission measurements. The effect of temperature and ionic strength of the medium have been employed to investigate the nature of the underlying interactions in the present host–guest system. The possibility of indicator displacement assay involving the present host–guest system has also been demonstrated using a competitive binder.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...