Gold(i)-catalyzed Nicholas reaction with aromatic molecules utilizing a bifunctional propargyl dicobalt hexacarbonyl complex†
Abstract
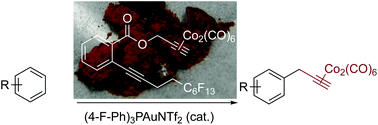
A benchtop-stable reagent for the catalytic Nicholas reaction was developed. By combining a propargyl dicobalt hexacarbonyl cluster with an ortho-alkynylbenzoate unit and a fluorous tag, introduction of a propargyl hexacarbonyl complex on various aromatic compounds having acid- or base-sensitive functional groups becomes possible by using a gold(I) catalyst. In addition, the presence of a fluorous tag facilitates convenient separation of the target products from byproducts.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...