Hydantoin analogs inhibit the fully assembled ClpXP protease without affecting the individual peptidase and chaperone domains†
Abstract
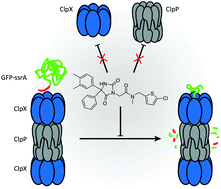
Proteolysis mediated by ClpXP is a crucial cellular process linked to bacterial pathogenesis. The development of specific inhibitors has largely focused on ClpP. However, this focus was challenged by a recent finding showing that conformational control by ClpX leads to a rejection of ClpP binders. Thus, we here follow up on a hit molecule from a high throughput screen performed against the whole ClpXP complex and demonstrate that stable inhibition with high potency is possible. Further investigations revealed that the small molecule binds to ClpP without affecting its activity. Likewise, the molecule does not inhibit ClpX and retains the overall oligomeric state of ClpXP upon binding. Structure activity relationship studies confirmed structural constraints in all three parts of the molecule suggesting binding into a defined stereospecific pocket. Overall, the inhibition of ClpXP without affecting the individual components represents a novel mechanism with perspectives for further optimization for in situ applications.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...