Traceless point-to-axial chirality exchange in the atropselective synthesis of biaryls/heterobiaryls
Abstract
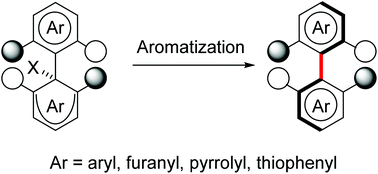
Traceless point-to-axial chirality exchange is a growing method for the preparation of axially chiral biaryls/heterobiaryls. During the exchange, stereogenic centers are destroyed and a chiral axis is simultaneously created. Highly functionalized enantiopure biaryls/heterobiaryls including those containing furanyl, thiophenyl, or pyrrolyl moieties with low rotation barriers have been prepared using this strategy with high levels of chirality transfer. The method is necessary for providing access to novel ligands, catalysts, natural products and active drugs having chiral axes. This review focuses on the chirality exchange approach for the formation of axially chiral biaryls/heterobiaryls and stereochemical models for the control of atropselectivity up to April 2019.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...