Hantzsch esters: an emerging versatile class of reagents in photoredox catalyzed organic synthesis
Abstract
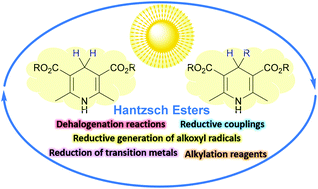
Hantzsch esters were often previously used as reductants in thermal catalytic hydrogenation reactions. Over the last few decades, Hantzsch esters have proven to be a useful class of electron donors and proton sources in photoredox catalyzed processes. Moreover, under photoredox catalytic conditions, alkyl-1,4-dihydropyridines can serve as versatile types of alkylation reagents via oxidative fragmentation mechanisms. This minireview highlights the recent advances in the chemistry of Hantzsch esters in photoredox catalyzed organic synthesis, with particular emphasis placed on reaction mechanisms. We hope that this review will inspire further new reaction design and developments with such a class of readily accessible reagents.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...