Fine structural tuning of styryl-based dyes for fluorescence and CD-based sensing of various ds-DNA/RNA sequences†
Abstract
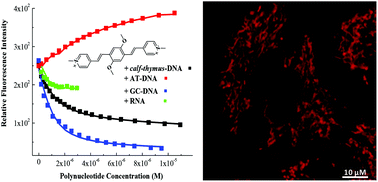
A set of styryl- and bis-styryl dyes, varying in length, aromatic surface, net positive charge and steric positioning or bulkiness of substituents, was tested for interactions with various ds-DNA or ds-RNA. Most of the compounds showed strong affinity toward ds-DNA/RNA, directly correlated to the synergistic contribution of the aromatic-conjugated surface and net positive charge. The volume or positioning of terminal aromatic substituents directly controlled the binding mode of the core structure, shifting between DNA/RNA groove binding or DNA/RNA intercalation. Consequently, upon binding to DNA/RNA the fluorimetric and induced CD (ICD) response varied for different compounds, for instance one derivative showed specific fluorescence increase with AT-DNA, while another derivative showed specific ICD response with AU-RNA. Preliminary screening on human tumour cell lines revealed an efficient cellular uptake for all dyes. Only mono-styryl-quinoline derivatives showed a strong antiproliferative activity combined with efficient fluorescent localisation, thus showing promising theragnostic potential, while other compounds were negligibly cytotoxic but still efficient fluorescent markers of cytoplasmic organelles.


 Please wait while we load your content...
Please wait while we load your content...