Improvement of the versatility of an arabinofuranosidase against galactofuranose for the synthesis of galactofuranoconjugates†
Abstract
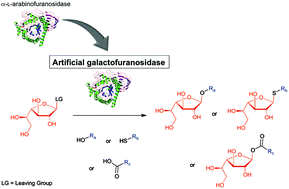
Galactofuranoconjugates are rare compounds with interesting biological properties. Their syntheses by traditional approaches are however tedious. Glycosidases are nowadays often used to simplify such syntheses but the use of galactofuranosidase has not been described yet for the synthesis of galactofuranoconjugates. Interestingly CtAraf51, an α-L-arabinofuranosidase from Ruminiclostridium thermocellum, is able to use aryl- or alkyl-β-D-galactofuranosides as the substrate but with very low efficiency. To allow its use as a synthesis tool, we decided to improve the efficiency of this enzyme toward these non-natural substrates. First, we identified three residues that can contribute to unfavorable interactions with the p-nitrophenyl-β-D-galactofuranoside. After mutagenesis, two mutants have shown a catalytic efficiency four- and threefold higher than that of the wild type, respectively. These two mutants were then evaluated in the transglycosylation reaction using ethanol as a model acceptor substrate. Under these conditions one mutant was much more efficient: 50% conversion was reached ten times faster than with the WT. Finally both mutants were converted into thioglycoligases: in the thioligation reaction, the reaction was two times faster than with the E173A single mutant, and in the acylation reaction a fourfold increase in the initial velocity was found. The synthetic potential of the resulting mutants to synthesize various O-, S- and acyl galactofuranoconjugates was further evaluated and yields up to 82% were obtained for the synthesis of ethyl- or thiophenyl galactofuranosides and methoxybenzoic galactofuranose.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...