Total syntheses of the bilirubin oxidation end product Z-BOX C and its isomeric form Z-BOX D†
Abstract
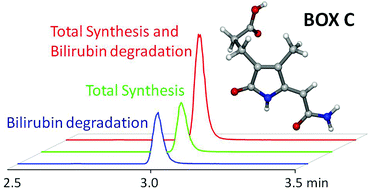
Oxidative degradation products of bilirubin (BOXes) are biologically highly active and certain BOXes cause long-lasting narrowing of cerebral blood vessels presumably with a significant role in subarachnoid hemorrhage. Due to the fact that mode of action as well as fate of these BOXes is widely unknown, larger amounts of these bilirubin degradation end products are required. The total synthesis of colorless (Z)-3-(5-(2-amino-2-oxoethylidene)-4-methyl-2-oxo-2,5-dihydro-1H-pyrrol-3-yl)propanoic acid (BOX C) succeeds via a seven-step procedure with a total yield of 20%. Its isomeric form (Z)-3-(2-(2-amino-2-oxoethylidene)-4-methyl-5-oxo-2,5-dihydro-1H-pyrrol-3-yl)propanoic acid (BOX D) can be prepared via a five-step protocol with a yield of 30%. NMR and crystallographic studies reveal that charge delocalization within the conjugated π-systems of BOXes C and D is negligible. Exposure of solutions of Z-BOX C and Z-BOX D to bright sunlight leads to Z/E-isomerization and mixtures of the respective E/Z-BOXes C and D.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...