Recent advances in catalytic asymmetric intermolecular oxidation of alkynes
Abstract
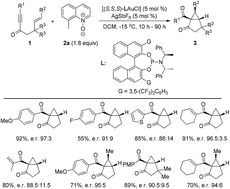
In recent years, gold-catalyzed intermolecular alkyne oxidation by an N-oxide oxidant, which presumably involves a gold carbenoid intermediate, has attracted increasing attention because it circumvents the employment of hazardous and potentially explosive diazocarbonyl compounds as starting materials for carbene generation. More importantly, the development of a catalytic enantioselective version of the reaction can help achieve an array of asymmetric synthesis processes modified from various racemic transformations of the gold carbenoid intermediate. In this review, we will present an overview of these recent advances in the asymmetric transformations by utilizing chiral ligands, chiral N,N′-dioxides and chiral substrates, aiming to facilitate progress in this fascinating field of research.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...