Mechanistic insights into ring cleavage of hydroquinone by PnpCD from quantum mechanical/molecular mechanical calculations†
Abstract
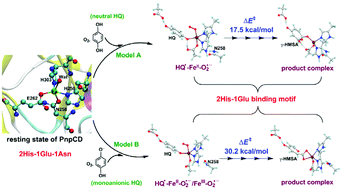
PnpCD is a mononuclear non-heme iron(II) dioxygenase containing an unusual 2His-1Glu-1Asn metal-binding motif. To gain insights into the catalytic mechanism of the ring opening of hydroquinone by PnpCD, hybrid quantum mechanics/molecular mechanics calculations have been performed by using two models with different protonation states of the substrate (nonionized and ionized forms of the Fe-bound hydroxyl group of hydroquinone). In both cases, the structure of the reactive Fe–O2 species reveals a trigonal bipyramidal complex, in which Asn258 is no longer coordinated to the iron center. The catalytic process mainly involves the attack of the superoxo radical, O–O bond cleavage, three-membered ring closure and opening, attack of the Fe-bound oxyl radical, and ring-opening of the seven-membered ring. The transition state for the peroxo O–O bond cleavage was found to be the rate-determining transition state. The second-sphere Glu248 serves as a proton acceptor to deprotonate the unbound substrate hydroxyl group, thereby facilitating the electron transfer between the substrate and dioxygen. The first-sphere Glu262 can act as an acid–base catalyst to lower the rate-limiting barrier, thus providing a useful clue for improving catalytic efficiency.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...