Functionalised bicyclic tetramates derived from cysteine as antibacterial agents†
Abstract
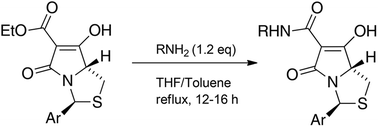
Routes to bicyclic tetramates derived from cysteine permitting ready incorporation of functionality at two different points around the periphery of a heterocyclic skeleton are reported. This has enabled the identification of systems active against Gram-positive bacteria, some of which show gyrase and RNA polymerase inhibitory activity. In particular, tetramates substituted with glycosyl side chains, chosen to impart polarity and aqueous solubility, show high antibacterial activity coupled with modest gyrase/polymerase activity in two cases. An analysis of physicochemical properties indicates that the antibacterially active tetramates generally occupy physicochemical space with MW of 300–600, clog D7.4 of −2.5 to 4 and rel. PSA of 11–22%. This work demonstrates that biologically active 3D libraries are readily available by manipulation of a tetramate skeleton.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...