Improved synthesis of the bifunctional chelator p-SCN-Bn-HOPO†
Abstract
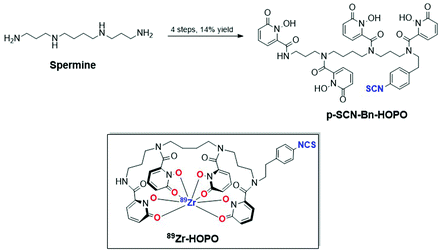
The bifunctional ligand p-SCN-Bn-HOPO, which has four 1,2-hydroxypyridinone groups on a spermine backbone with an isothiocyanate linker, has been shown to be an efficient and stable chelator for Zr(IV) and, more importantly, the radioisotope 89Zr for use in radiolabeling antibodies for positron emission tomography (PET) imaging. Previous studies of 89Zr-HOPO-trastuzumab in mice showed low background, good tumor to organ contrast, and very low bone uptake which show p-SCN-Bn-HOPO to be an important next-generation bifunctional chelator for radioimmunoPET imaging with 89Zr. However, the reported synthesis of p-SCN-Bn-HOPO involves nine steps and multiple HPLC purifications with an overall yield of about 1.4%. Herein we report an improved and efficient synthesis of p-SCN-Bn-HOPO in four steps with 14.3% overall yield which will improve its availability for further biological studies and wider application in PET imaging. The new synthetic route also allows variation in linker length and chemistries which may be helpful in modifying in vivo clearance behaviors of future agents.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...