Photoaffinity palladium reagents for capture of protein–protein interactions†
Abstract
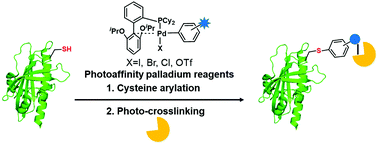
Protein–protein interactions (PPIs) are indispensable in almost all cellular processes. Probing of complex PPIs provides new insights into the biological system of interest and paves the way for the development of therapeutics. Herein, we report a strategy for the capture of protein–protein interactions using photoaffinity palladium reagents. First, the palladium-mediated reagent site specifically transferred a photoaffinity modified aryl group to the designated cysteine residue. Next, the photoaffinity group was activated by UV radiation to trap the proximal protein residue for the formation of a crosslink. This strategy was used to capture the PYL-ABA-PP2C interaction, which is at the core of the abscisic acid (ABA) signalling pathway. Our results indicated that this palladium-mediated strategy can serve as an alternative for incorporating an increasing number of diverse substrates for protein crosslinking through cysteine modifications and can be explored for use in mapping protein–peptide or protein–protein interaction surfaces and in trapping potential interacting partners.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...