Visible-light mediated sulfonylation of thiols via insertion of sulfur dioxide†
Abstract
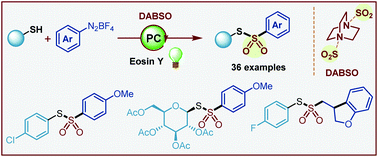
A simple and efficient visible-light mediated synthesis of thiosulfonates via a radical–radical coupling of sulfenyl radicals and arylsulfonyl radicals was developed. The reaction of thiols, aryldiazonium tetrafluoroborates and DABSO proceeded at room temperature using 5 mol% eosin Y. The reaction displayed wide functional group tolerance and delivered the unsymmetrical thiosulfonates in good to excellent yields.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...