Ring closing metathesis (RCM) approach to the synthesis of conduramine B-2, ent-conduramine F-2, aminocyclopentitol and trihydroxyazepane†
Abstract
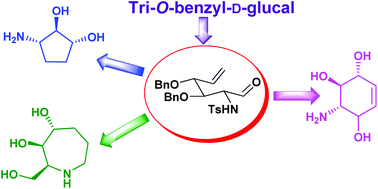
The syntheses of conduramine B-2, ent-conduramine F-2, aminocyclopentitol and trihydroxyazepane were accomplished from a common precursor, through a divergent approach using ring closing metathesis (RCM) as the key step. Tri-O-benzyl-D-glucal was converted to 3,4,6-tri-O-benzyl-1,2-dideoxy-2-iodo-1-p-toluenesulfonamido-α-D-mannose. Exposure to NaBH4 in MeOH resulted in a facile 1,2-transposition of the –NHTs group with concomitant glycosylation to give methyl 3,4,6-tri-O-benzyl-2-deoxy-2-p-toluenesulfonamido-β-D-glucoside, which was converted into methyl 6-deoxy-6-iodo-glucoside in three steps. Zinc-mediated Vasella's rearrangement proceeded smoothly to give the pluripotent formyl-olefin, possessing both electrophilic and nucleophilic sites, which was used as a common precursor in our diversity-oriented approach. Vinylation of the carbonyl group followed by RCM and subsequent deprotection resulted in the successful synthesis of conduramine B-2 and ent-conduramine F-2 for the first time. On the other hand, the Wittig reaction of the formyl-olefin affords the diene that undergoes Grubbs’ I catalyzed RCM and deprotection/reduction to provide 3-amino-cyclopentan-1,2-diol. Utilizing the nucleophilic site at the nitrogen of the common precursor, base mediated N-allylation was carried out to obtain the corresponding diene that underwent a smooth RCM to afford trihydroxyazepane.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...