Cu(i) catalysed annulation of isothiocyanates/isocyanates with 2-iodo-sulfonamides: synthesis of benzodithiazines, benzothiadiazinones, benzothiazinylidene-anilines and benzothiazolylidene-anilines†
Abstract
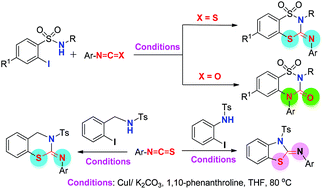
An efficient Cu(I)-catalysed cyclisation reaction of 2-iodobenzene sulfonamides with aryl-isothiocyanates and isocyanates that affords functionalised benzodithiazines and benzothiadiazinones, respectively, has been developed. Thus, in the reaction with aryl isothiocyanates (Ar–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), the C
S), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S moiety participates in the cyclisation leading to a dithiazine. By contrast, in the case of aryl isocyanates (Ar–N
S moiety participates in the cyclisation leading to a dithiazine. By contrast, in the case of aryl isocyanates (Ar–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), the N
O), the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C part is involved in the cyclisation and a thiadiazinone is obtained. Analogous reactions of isothiocyanates with N-tosyl-2-iodo-anilines and 2-iodo-benzyl sulfonamides afford (benzothiazin-2-ylidene)anilines and (benzothiazol-2-ylidene)anilines, respectively. Probable mechanistic pathways are briefly discussed.
C part is involved in the cyclisation and a thiadiazinone is obtained. Analogous reactions of isothiocyanates with N-tosyl-2-iodo-anilines and 2-iodo-benzyl sulfonamides afford (benzothiazin-2-ylidene)anilines and (benzothiazol-2-ylidene)anilines, respectively. Probable mechanistic pathways are briefly discussed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...