Abstract
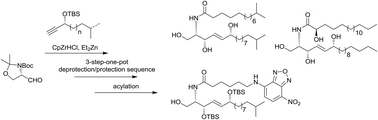
6-Hydroxy-(4E)-sphingenine-containing sphingolipids are found in mammalian and bacterial membranes and have multiple intra- and intercellular functions. Most sphingolipids contain a (2S,3R)-2-amino-1,3-diol core structure, but only limited examples of unnatural (2S,3S)-2-amino-1,3-diol derivates have so far been reported. Using an underexplored hydrozirconation–transmetalation reaction and an unusual three-step-one-pot deprotection sequence, we were able to synthesize several unnatural (2S,3S)-6-hydroxy-(4E)-sphingenine-containing sphingolipids in only three (protected) or four (deprotected) consecutive steps, respectively, including a fluoresence-labeled derivative suitable for future biological studies.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...