A detailed mechanism of the oxidative half-reaction of d-amino acid oxidase: another route for flavin oxidation†
Abstract
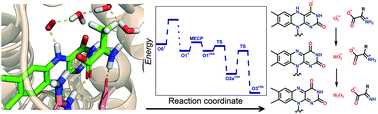
D-Amino acid oxidase (DAAO) is a flavoenzyme whose inhibition is expected to have therapeutic potential in schizophrenia. DAAO catalyses hydride transfer from the substrate to the flavin in the reductive half-reaction, and the flavin is reoxidized by O2 in the oxidative half-reaction. Quantum mechanical/molecular mechanical calculations were performed and their results together with available experimental information were used to elucidate the detailed mechanism of the oxidative half-reaction. The reaction starts with a single electron transfer from FAD to O2, followed by triplet–singlet transition. FAD oxidation is completed by a proton coupled electron transfer to the oxygen species and the reaction terminates with H2O2 formation by proton transfer from the oxidized substrate to the oxygen species via a chain of water molecules. The substrate plays a double role by facilitating the first electron transfer and by providing a proton in the last step. The mechanism differs from the oxidative half-reaction of other oxidases.


 Please wait while we load your content...
Please wait while we load your content...