Selectfluor-promoted regioselective chlorination/bromination of 2-aminopyridines and 2-aminodiazines using LiCl/LiBr†
Abstract
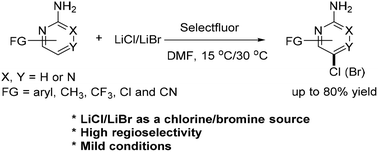
Using LiCl as a chlorine source, the chlorination of 2-aminopyridines or 2-aminodiazines in the presence of Selectfluor and DMF is established under mild conditions. This method gives chlorinated pyridines or diazines in good to high yields with high regioselectivities. Also, this method is extended to the bromination of 2-aminopyridines or 2-aminodiazines by using LiBr. The regioselectivity of the chlorination reaction is strongly dependent upon the substituent pattern in either the 2-aminopyridines or 2-aminodiazines. The synthesis of Buparlisib from chlorinated pyridines was explored. A study of the mechanism revealed that this chlorination occurs via either a pyridine or diazine radical process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...