Ene reactions of 2-borylated α-methylstyrenes: a practical route to 4-methylenechromanes and derivatives†
Abstract
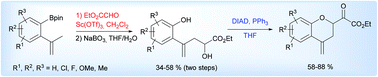
4-Methylenechromanes were prepared via a three-step process from 2-borylated α-methylstyrenes. This sequence is based on a key glyoxylate-ene reaction catalyzed by scandium(III) triflate. The resulting γ-hydroxy boronates, which cyclise to seven-membered homologues of benzoxaborole on silica gel, were cleanly oxidized with sodium perborate, and then cyclised under Mitsunobu conditions. Additionally, several further functional transformations of 4-methylenechromanes or their precursors were carried out to illustrate the synthetic potential of these intermediates.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...