Gold(i)-promoted α-selective sialylation of glycosyl ortho-hexynylbenzoates for the latent-active synthesis of oligosialic acids†
Abstract
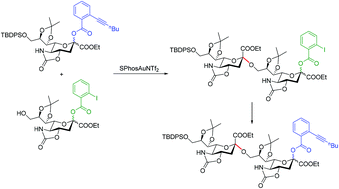
A gold(I)-promoted α-selective sialylation approach with 5-N,4-O-oxazolidinone-protected sialyl ortho-hexynylbenzoates as donors is described for the stereoselective synthesis of α-sialosides. Iterative couplings of the ‘active’ sialyl ortho-hexynylbenzoates and the ‘latent’ sialyl ortho-iodobenzoates provide a new approach for the ‘latent-active’ synthesis of α-(2 → 9)-linked oligosialic acids that are relevant to N. meningitidis serogroup C capsular polysaccharide.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...