Bianthryl-based organocatalysts for the asymmetric Henry reaction of fluoroketones†
Abstract
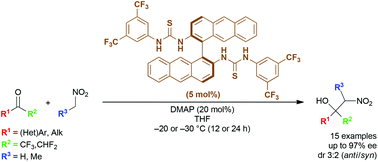
We have developed a catalytic system based on bianthrylbis(thiourea) for the asymmetric Henry reaction of fluoroketones and nitroalkanes that resulted from the screening of a library containing 31 chiral non-racemic organocatalysts. The corresponding adducts were isolated in up to 6 times shorter reaction time in comparison with the previously published organocatalysts. High levels of stereocontrol have been generally observed, with measured product enantiomeric excesses up to 97% and diastereomeric ratio 3 : 2 (anti/syn). The above-mentioned catalysts have been successfully applied to the total asymmetric synthesis of CF3-tethered (S)-halostachines, which has proved that this method constitutes an easy entry to similar enantiopure compounds.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...