Total syntheses of several iridolactones and the putative structure of noriridoid scholarein A: an intramolecular Pauson–Khand reaction based one-stop synthetic solution†
Abstract
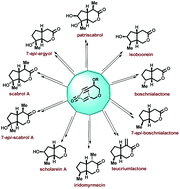
A simple and general approach towards the total syntheses of several iridolactones such as (±)-boschnialactone, (±)-7-epi-boschnialactone, (±)-teucriumlactone, (±)-iridomyrmecin, (±)-isoboonein, (±)-7-epi-argyol, (±)-scabrol A, (±)-7-epi-scabrol A, and (±)-patriscabrol as well as the putative structure of scholarein A is delineated. The synthetic strategy features a diastereoselective intramolecular Pauson–Khand reaction (IPKR) to construct the iridoid framework followed by some strategic synthetic manipulations to access the targeted monoterpenes including those having diverse oxy-functionalization patterns and with 3–5 contiguous stereogenic centres in a highly stereocontrolled manner. Also, the present endeavour includes the first total synthesis of scabrol A.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...